NRF2 is just one of thousands of critical proteins in the cell, but it is one that we now know a lot about. Once any molecule achieves a certain level of celebrity status, it tends to acquire a groupie following in the supplement market. Today, we have all manner of NRF enhancers, releasers, activators and synergizers ready to arrive on your doorstep at the click of a button. But what could any of these things possibly do for us, and how much is too much of a good thing?
At the risk of overstating the obvious, if a little extra NRF2 is good for every cell in your body, and every cell in your body is good, then NRF2 must be good for your body. The weak link in that argument, however, is that all cells are not good. Nobody wants harmful bacterial cells to flourish, and nobody wants cancer cells to flourish. A paper recently published in Nature now suggests that inhibiting NRF2 can block the migration and invasion of non-small-cell lung cancer cells through the body. If anyone is going to derive benefit from NRF2, they may need to be smart about it.
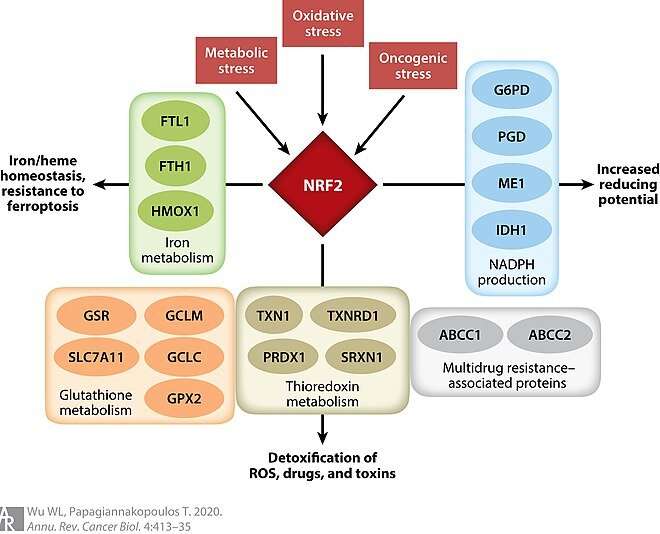
The main reason NRF2, or Nuclear factor-erythroid 2-related factor 2, is so highly sought, is because it is a key transcriptional regulator of several antioxidant and anti-inflammatory enzymes. Unfortunately, as the authors above have revealed, it also moonlights as an activator of the Rho-ROCK pathway, which promotes actin filamentation and movement of cells. The researchers were able to block this activity of NRF2 by giving an inhibitor known as brusatol.
By now many people appreciate that viruses, bacteria and parasites have complex life cycles with various maturation waypoints within their hosts. Proteins, although they are typically confined within or on a cell, also have complicated life cycles. In this sense, our expansive knowledge of the larger NRF2 ecosystem permits us a convenient microcosm of the cell. For example, soon after NRF2 is made by ribosomes in the cytoplasm, it is normally sequestered by KEAP1, which quickly loops in the ubiquitin ligase Cullin3 for transport to the proteasome. Here, the ubiquitin is stripped off and NRF2 is degraded and recycled. If all is well in the cell, this process gives NRF2 a half life of about 20 minutes.
However, under oxidative or electrophilic stress, reduced cysteine residues in Keap1 are oxidized, ultimately blocking the ubiquitination cycle. As NRF2 concentration increases, it translocates to the nucleus, forms heterodimers, and binds the promoters of antioxidant genes to increase their expression. This particular set of genes, termed the NRF2 regulon, include modulators of drug metabolism, stress response, iron metabolism and excretion/transporter, and glutathione homeostasis. Glutathione keeps a normally protective apoptotic process called ferroptosis in check.
When cells don’t have enough cysteine to make glutathione, oxidation of membrane lipids go unrepaired and ferroptosis eliminates the cell. Glutamate cysteine ligase catalyzes ATP-dependent condensation of glutamate and cysteine in the first and rate-limiting step of glutathione synthesis. One of the main functions of NRF2 is the induction of glutamate cysteine ligase, and recent research indicates that this ligase protects against ferroptosis by a non-canonical mechanism, causing accumulation of γ-glutamyl-peptides.
Source: Read Full Article
