Vaccine-conferred immunity against the coronavirus disease 2019 (COVID-19) wanes with increasing time after vaccination. To enhance this immunity, a third booster dose of current COVID-19 vaccines has been recommended.
Study: Acquired neutralizing breadth against SARS-CoV-2 variants including Omicron after three doses of mRNA COVID-19 vaccination and the vaccine efficacy. Image Credit: Billion Photos / Shutterstock.com
To assess the efficacy of a booster dose, a team of scientists from the Kobe University Graduate School of Medicine, Japan, investigated the induction of neutralizing antibodies against D614G, Alpha, Beta, Gamma, Delta, Kappa, and Omicron variants of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
The researchers of the current study, which is published on the medRxiv* preprint server, also analyzed the neutralizing antibody levels against the SARS-CoV-2 D614G, Delta, and Omicron strains in individuals after receiving the booster dose.
Booster vaccination
Since the original SARS-CoV-2 wild-type strain was detected in December 2019, several other variants of concern (VOCs) have emerged, which include the Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), and Omicron (B.1.1.529) variants. The Omicron variant is the most recently identified SARS-CoV-2 VOC that was detected in early November 2021.
Vaccine efficacy is negatively impacted by SARS-CoV-2 VOCs to varying extents. Furthermore, neutralizing antibody responses induced by COVID-19 vaccines decrease with increased time post-vaccination.
In the United States, a third booster dose has been approved for fully vaccinated individuals after six months of the last dose. Recent studies have found that the booster dose can boost immunity and induce high levels of neutralizing antibodies; however, there remains limited information on whether the immune response mounted by the booster dose is effective against the Omicron variant.
About the study
A total of 82 individuals were fully vaccinated with the Pfizer-BioNTech BNT162b2 messenger ribonucleic acid (mRNA) COVID-19 vaccine and never tested positive for the coronavirus disease 2019 (COVID-19) were selected for this study. Of all the participants of the study, 72 individuals received booster vaccination.
Blood samples were collected and analyzed at two and seven months after the second dose and 16 days after the third dose. Samples were also collected from 24 unvaccinated healthy individuals as controls.
The neutralizing activity against SARS-CoV-2 variants was assessed using the neutralization assay. The SARS-CoV-2 D614G strain, as well as the Alpha, Beta, Gamma, Delta, Kappa, and Omicron variants, were used in this study.
To study the effect of age on antibody levels, the participants were divided into three groups by age. Study participants 38 years and younger were classified as the younger group; 39–58-year-olds were classified as the middle-aged group; and those who were 59 years and older were classified as the older group. There were 31 participants (37.8%) in the younger group, 32 participants (39.0%) in the middle-aged group, and 19 participants (23.2%) in the older group. Taken together, 86.6% of participants were males.
Higher antibody levels after the booster dose
When the neutralizing antibody levels were estimated two months after the second dose, all participants had neutralizing activity against D614G and Alpha variants. However, the antibody levels varied.
Serum samples from 87.8%, 95.1%, 92.7%, and 90.2% of the participants showed neutralizing activity against the Beta, Gamma, Delta, and Kappa variants, respectively. Comparatively, only 28% of the participants showed neutralizing activity against the Omicron variant. Furthermore, antibody levels were 11.8-fold, 7.3-fold, 3.2-fold, 4.9-fold, 3.6-fold, and 2.6-fold lower than those against D614G, Alpha, Beta, Gamma, Delta, and Kappa variants, respectively.
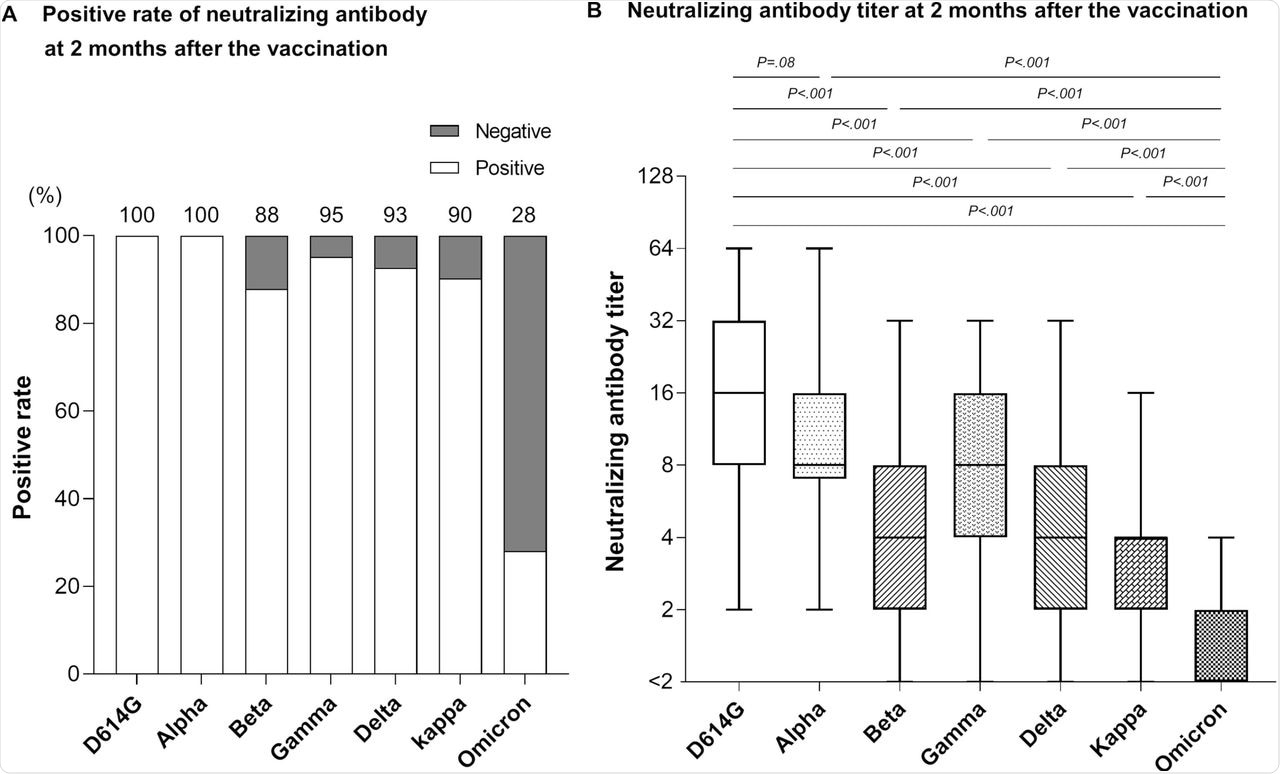
Neutralizing antibody against SARS-CoV-2 variants at 2 months post-vaccination.
When neutralizing antibody levels were estimated seven months after the second dose, 93% and 67% of the participants showed neutralizing activity against D614G and Delta variants, respectively. The neutralizing antibody levels were 2.2-fold and 2.1-fold lower for D614G and Delta variants, respectively.
Only 6% of participants showed neutralizing activity against the Omicron variant. This increased to 100% after the third booster dose. Furthermore, neutralizing antibody levels were 32-fold and 39-fold higher than the levels at two- and seven-months post-vaccination.
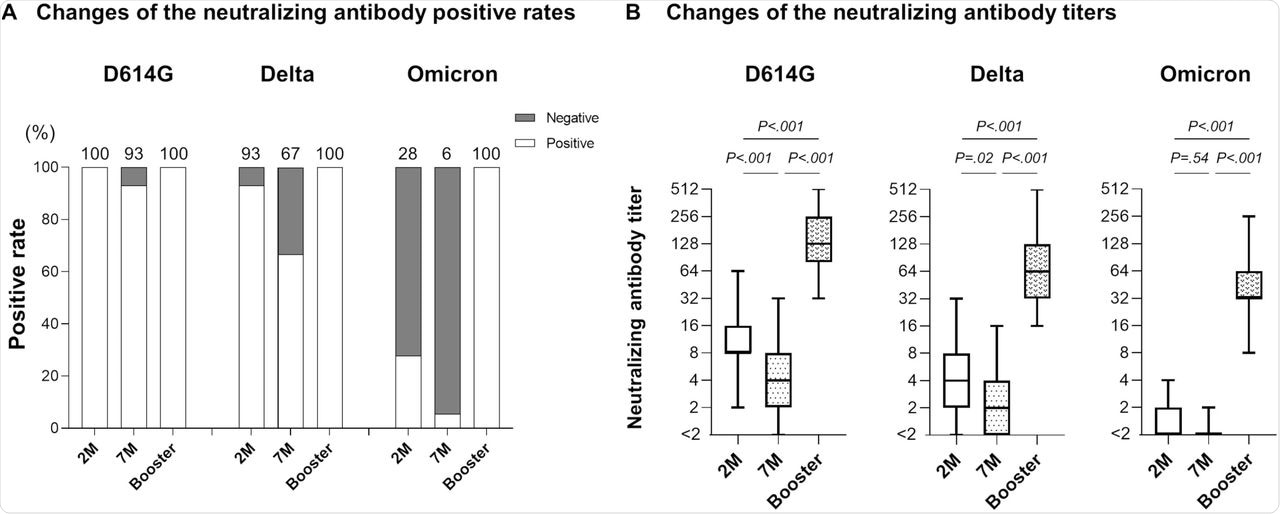
Neutralizing antibody after booster vaccination.
For the other variants, the neutralizing antibody level increased at two- and seven-months post-vaccination were 13-fold and 31-fold for D614G, respectively, and 16-fold and 35-fold for Delta variants, respectively.
When the participants were stratified into different age groups, the rise in neutralizing antibody levels was 41-fold for younger participants, 43-fold for middle-aged participants, and 27-fold for older participants. These antibody levels after the booster dose were higher than the predicted levels.
Conclusions
According to the findings from the current study, two doses of mRNA vaccines are not sufficient to confer immunity against the Omicron variant.
Neutralizing antibody levels were estimated at 16 days after the third dose; therefore, further research is needed to determine whether these levels wane in the same manner that levels wane months after the second dose.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Furukawa, K., Tjan, L. H., Kurahashi, Y., et al. (2022). Acquired neutralizing breadth against SARS-CoV-2 variants including Omicron after three doses of mRNA COVID-19 vaccination and the vaccine efficacy. medRxiv. doi:10.1101/2022.01.25.22269735. https://www.medrxiv.org/content/10.1101/2022.01.25.22269735v2.
Posted in: Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: Antibodies, Antibody, Assay, Blood, Coronavirus, Coronavirus Disease COVID-19, covid-19, Efficacy, Immune Response, immunity, Medicine, Omicron, Research, Respiratory, Ribonucleic Acid, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine

Written by
Dr. Shital Sarah Ahaley
Dr. Shital Sarah Ahaley is a medical writer. She completed her Bachelor's and Master's degree in Microbiology at the University of Pune. She then completed her Ph.D. at the Indian Institute of Science, Bengaluru where she studied muscle development and muscle diseases. After her Ph.D., she worked at the Indian Institute of Science, Education, and Research, Pune as a post-doctoral fellow. She then acquired and executed an independent grant from the DBT-Wellcome Trust India Alliance as an Early Career Fellow. Her work focused on RNA binding proteins and Hedgehog signaling.
Source: Read Full Article