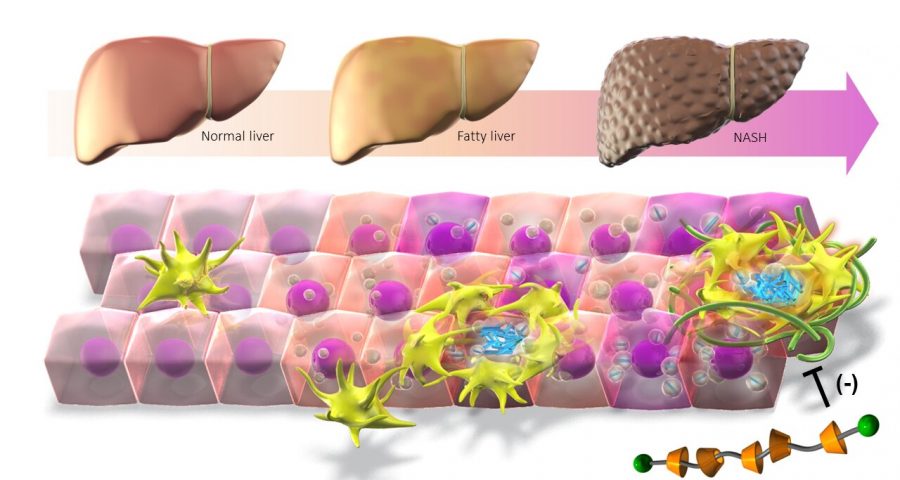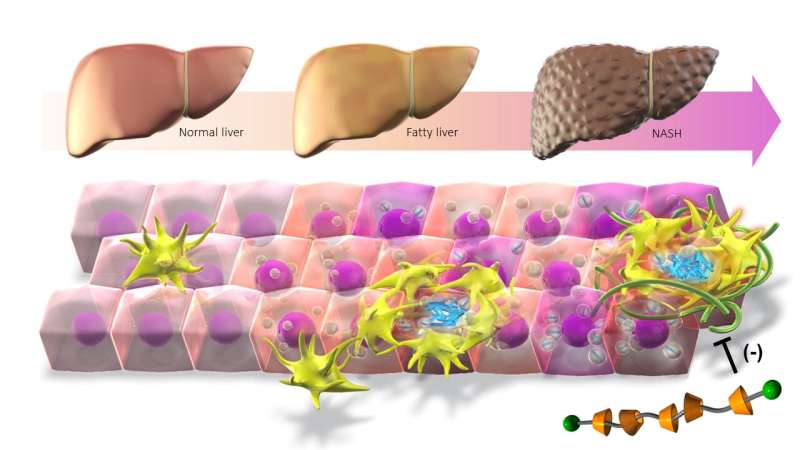
A research group from the Graduate School of Medicine and Research Institute of Environmental Medicine at Nagoya University report that cholesterol accumulation in macrophages promotes liver fibrosis in the development of non-alcoholic steatohepatitis (NASH).
Using a unique supramolecule, they removed cholesterol in a mouse model, stopping the development of the disease. As cholesterol crystals are also found in human patients, this suggests a potential treatment for the disease. Their findings were published in the Journal of Experimental Medicine.
As the number of patients with obesity increases, so do cases of fatty liver. Fatty liver risks progressing to NASH, a severe disease characterized by inflammation, fibrosis, and the increased death of the functional cells in the liver known as hepatocytes. Although it is known that cholesterol accumulation promotes NASH development, the precise mechanism is unknown.
Macrophages, the body’s immune cells, are also found around the dead hepatocytes, attracted to the inflamed liver tissue by signals released by damaged liver cells. Hepatocytes release cytokines, which amplify the inflammation, leading to tissue damage and fibrosis.
To further understand the mechanism, the group led by Nagoya University doctors Takayoshi Suganami and Michiko Itoh used a combination of techniques to identify cholesterol crystals in the lipids of the dead hepatocytes and the macrophages that engulf the cells.
To understand whether the cholesterol was involved in NASH, the researchers needed to find out if removing it improved the symptoms. To do this, they synthesized a unique supramolecule that combined the oligosaccharide β-cyclodextrin, which encapsulates free cholesterol, with a polymer to create β-cyclodextrin polyrotaxane (βCD-PRX).
When they administered the supramolecule to mice, they found that it accumulated in the liver where it promoted excretion of cholesterol, which effectively stopped the progression of NASH.
“Cholesterol crystals are also observed in human NASH patients, which suggests that βCD-PRX could be a potential therapeutic strategy for human NASH,” Suganami said.
“Recent advances in analytical techniques have led us to understand the characteristics of macrophages, which play crucial roles in the development of NASH. This study provides evidence that cholesterol overload triggers macrophage changes and promotes the development of NASH, which could be a novel therapeutic target.”
More information:
Michiko Itoh et al, Lysosomal cholesterol overload in macrophages promotes liver fibrosis in a mouse model of NASH, Journal of Experimental Medicine (2023). DOI: 10.1084/jem.20220681
Journal information:
Journal of Experimental Medicine
Source: Read Full Article