A university researcher is studying the potential effect of leaky gut syndrome on our overall health. “A mechanism by which gut microbiota elevates permeability and inflammation in obese/diabetic mice and human gut,” a paper coauthored by Bo Wang, chemical engineering assistant professor, and researchers at the University of South Florida, looks at the role of gut microbes. The paper was published in the spring edition of Gut.
Gut microbiota is the collection of bacteria and other microorganisms that live in the digestive tract. 100 trillion micro-organisms (most of them bacteria, but also viruses, fungi, and protozoa) exist in the human gastrointestinal tract and plays a role in nutrient metabolism, drug metabolism and protection against pathogens.
The paper also examines metabolomics, which is the study of small molecules (usually known as metabolites) within cells, biofluids, tissues or organisms. Together, these small molecules and their interactions within a biological system are known as the metabolome.
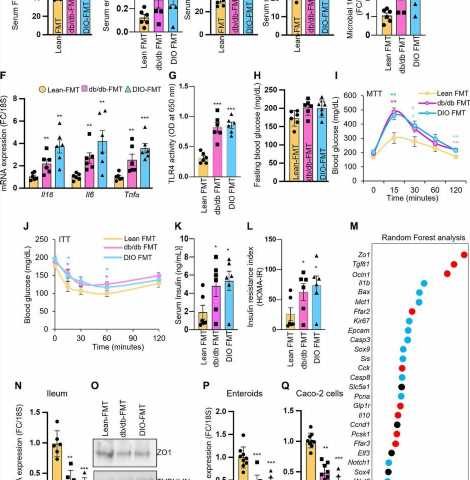
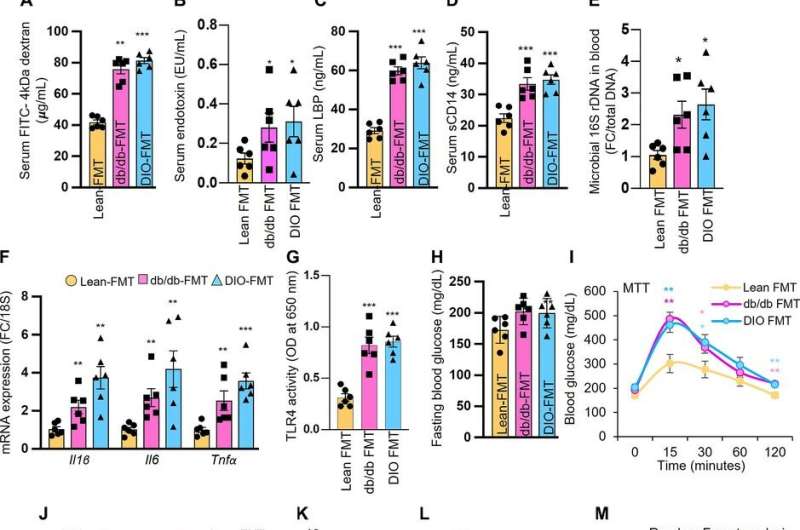
Intestinal permeability, also known as leaky gut syndrome, is a theoretical condition where the intestines leak water and nutrients. That can lead to inflammation and changes in the normal gut bacterial which in turn could cause problems within the digestive tract.
The study was conducted by examining the metabolites of mice. He would mirror them against other mice metabolites to see how many of them were present and their relative conditions. Wang and his collaborators analyzed the differences between metabolites from obese or diabetic mice and non-diabetic mice for control. They found that microbiota of both obese humans and mice reduced ethanolamine-metabolizing capacity in the gut, which naturally led to increased ethanolamine accumulation.
While ethanolamine naturally occurs in the gut, obesity can lead to the gut accumulating higher levels of it, which in turn can instigate leaky gut syndrome, inflammation and impaired glucose metabolism, the study found.
The study also found that two weeks of a meat-supplemented diet for the mice also promoted ethanolamine accumulation by reducing microbiota’s ethanolamine-metabolizing capacity.
A possible solution? Restoring the ethanolamine-metabolizing function of gut microbiota using human-origin probiotic therapy. Wang and his collaborators were able to reverse elevated gut permeability, inflammation and dysfunction of glucose metabolism using lactobacillus rhamnosus, a natural bacteria in the gut that is also available as a dietary supplement.
“This probiotic therapy, they actually add some good bacteria inside of the guts,” Wang said. “Basically they feed the mouse those kind of good bacteria, and then try to balance the microbiome status inside the mouse, and try to see if we can overcome some gut leakage and reduce diabetes and obesity.”
Wang believes studies like this one can provide deeper insight into the role gut bacteria plays in diabetes, obesity and even Alzheimer’s disease, which Wang and his collaborators are examining in a separate study that looks to build upon independent research showing Alzheimer’s disease is related to gut bacteria.
More information:
Sidharth P Mishra et al, A mechanism by which gut microbiota elevates permeability and inflammation in obese/diabetic mice and human gut, Gut (2023). DOI: 10.1136/gutjnl-2022-327365
Journal information:
Gut
Source: Read Full Article