New treatment option against eczema successfully tested
Atopic diseases is a chronic skin, their dissemination has increased significantly in recent decades. Although mild forms of the disease today is often a relatively good external treatments to get a grip, but in the case of severe eczema little hope for those Affected at this stage. However, this could change thanks to a new Form of therapy soon.
“The disease affects about eleven percent of all girls and boys of preschool age, as well as one to two percent of adults in Germany, many of the disease is chronic and runs hard,” reports the Hannover Medical school (MHH). Affected suffer from dry, scaly and reddened skin, the agonizing itch and if the affected areas are well visible, also a social stigma. Effective treatment options are therefore urgently needed – however, these were not available for the severe forms of the disease so far. Researchers at the MHH and the University of Veterinary medicine Hannover (TiHo) have now tested successfully a new approach. Their results were published in the specialist magazine “Journal of Allergy and Clinical Immunology”.
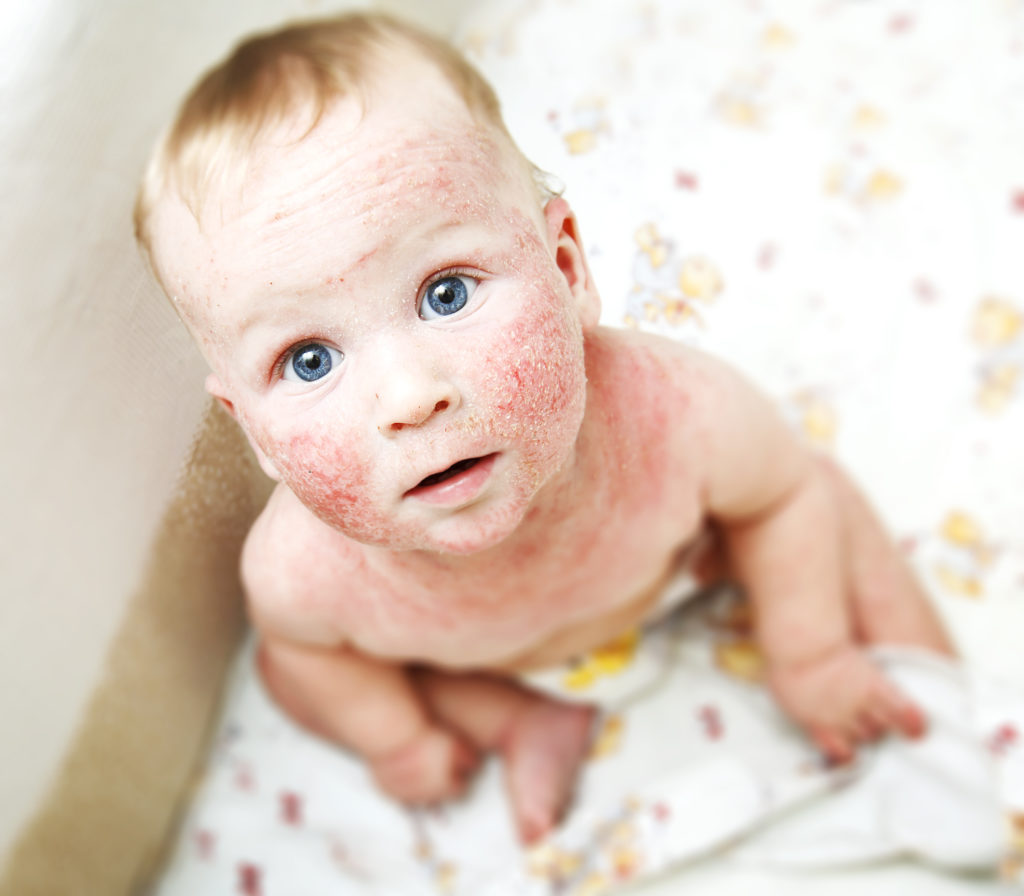
Severe eczema limited to treat
“Eczema has different causes, to the skin where irritation include substances, allergens as well as microbial, hormonal, and psychological influences”, the experts explain. In the treatment to be applied cortisone Compounds, and also the so-called Calcineurin inhibitors, to come so far, especially externally, are of Central importance. For the treatment of particularly serious forms of the immunosuppressant cyclosporine, which however has many side effects, and the antibody Dupilumab, according to the statements of the experts so far.
Dupilumab in the application a little difficult
Dupilumab for about a year for the targeted inhibition of messenger of allergic inflammation substances available, and “represents a very great advance in the treatment of severely affected patients”, says Professor Dr Thomas Werfel from the MHH clinic for dermatology, Allergology and venereology. However, it does not help all patients well enough. In addition, the drug should be injected, what will be tolerated, especially children, suffer especially common in atopic dermatitis, severe. The well tested a new active ingredient intended for oral ingestion.
New active substance for oral application
The new active ingredient, can be administered orally as a tablet, have improved in the tests of 98 patients, the appearance of the skin significantly. “After eight weeks, the proportion of diseased skin such as redness, blisters, and scratch marks reduced by half”, so the message of the MHH. The active ingredient is a “histamine-4-Receptor-Blocker”. This is to interrupt the inflammatory process and ease the itching by preventing the neurotransmitter histamine can act on the corresponding cells.
Histamine-4-Receptor with a key role
“Laboratory and In-vivo results in the mouse model, which we published continuously since 2005, for example, that the histamine-4-Receptor is an interesting target structure for the treatment of atopic dermatitis,” explains Professor Dr. Werfel. Since then the researchers have the application investigated in inflammatory skin diseases-intensive. “We assume that the histamine-4-Receptor-blockers, regardless of the cause of the eczema and are currently investigating which patients can benefit the most from the new therapy,” said Professor Werfel.
No side effects noticeable
In the current study were observed according to the researchers no side effects to the administration of the drug and will start with the participation of the team from Hanover, a larger international study with approximately 400 patients, the optimal dosage to find out of this drug. “We are working already since many years together on the topic. The project is a very good example for translational research, for an interdisciplinary medical research, with the objective to transfer the results as quickly as possible into clinical application,“ says Professor Dr. Manfred Kietzmann from the Institute of pharmacology, toxicology and pharmacy, TiHo. (fp)
