A multi-state study from the U.S. Center for Disease Control and Prevention’s (CDC) VISION Network has found that first-generation COVID-19 mRNA vaccines were associated with protection against COVID-19 during periods of omicron BA.4/BA.5 predominance.
The new analysis found that mRNA vaccines were protective against COVID-19-associated hospitalization and ICU admission or in-hospital death and noted less severe disease during BA.4/BA.5 predominance compared to earlier omicron variants.
During BA.4/BA.5 predominance, estimated 3-dose vaccine effectiveness against hospitalization was 68 percent between 7- and 119-days post-vaccination. Vaccine effectiveness against hospitalization decreased to 36 percent by 120 days or more post-vaccination.
For hospitalization, an indicator of severe disease, estimated vaccine effectiveness of three doses for all adults and four doses for adults aged 50 or older was similar to that reported during predominance of earlier omicron variants. However, hospitalized adults were less likely to be admitted to the ICU or experience in-hospital death and had shorter length of stay during BA.4/BA.5 predominance compared to earlier omicron variants.
“Our findings that COVID-19 mRNA vaccines remain effective and that vaccinated adults weren’t as sick during BA.4/BA.5 predominance is encouraging as we look ahead to development of future vaccines and strategies for combatting the virus,” said study co-author Shaun Grannis, M.D., M.S., Regenstrief Institute vice president for data and analytics.
“Estimation of COVID-19 mRNA Vaccine Effectiveness and COVID-19 Illness and Severity by Vaccination Status During omicron BA.4 and BA.5 Sublineage Periods,” is published in JAMA Network Open.
The study authors note that COVID-19 vaccine effectiveness estimation “has become complex as additional vaccine booster doses are authorized, vaccine-induced protection wanes over time, new variants or subvariants have emerged, and a majority of the US population has experienced previous infection (57-94%, depending on source).”
“As new variants emerge, ongoing monitoring of vaccine effectiveness is critical for informing public health strategies and treatment of patients,” said study co-author Brian Dixon, Ph.D., MPA, interim director of Regenstrief Institute’s Center for Biomedical Informatics. “Tracking performance of vaccines will also help develop better vaccines and inform best practices for future pandemics.”
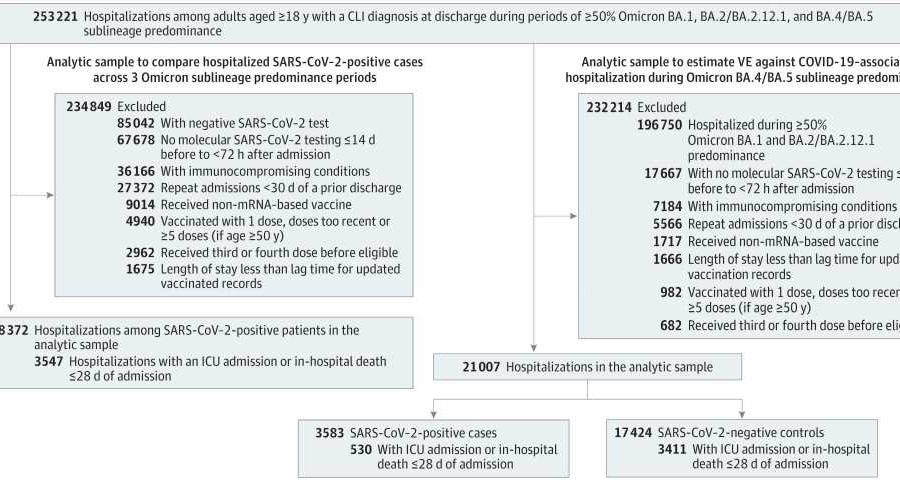
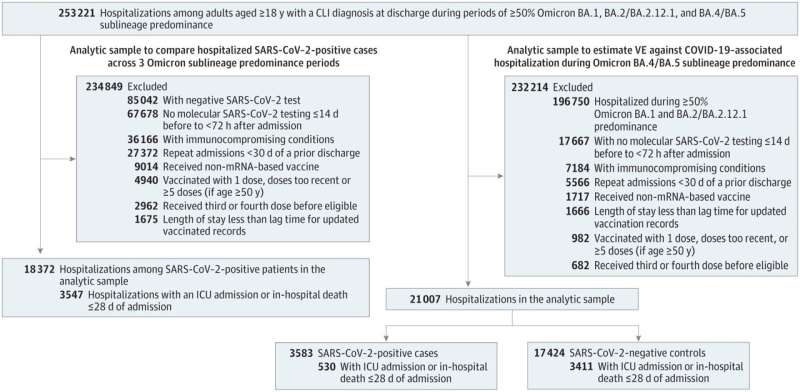
This new study analyzed 82,229 emergency department or urgent care encounters and 21,007 hospitalizations for COVID-19-like illness in adults ages 18 and older. Nine VISION sites in 10 states contributed data including 268 hospitals, 292 emergency departments and 140 urgent care clinics.
The authors write, “Estimated vaccine was similar across outcomes, contradicting many past vaccine effectiveness studies, including previous studies from VISION, which have tended to show higher vaccine-associated protection for more severe outcomes. This could be due to changes in baseline population immunity (e.g., most adults now have evidence of prior infection), changes in behavior (e.g., decreased use of social distancing and masks during recent months), or residual confounding.”
More information:
Estimation of COVID-19 mRNA Vaccine Effectiveness and COVID-19 Illness and Severity by Vaccination Status During Omicron BA.4 and BA.5 Sublineage Periods, JAMA Network Open (2023). DOI: 10.1001/jamanetworkopen.2023.2598
Journal information:
JAMA Network Open
Source: Read Full Article